The culturing and preparation of cells or tissues generates liquid wastes that must be managed according to the biological, chemical, and radiological hazards they contain. Potentially infectious cell or tissue culture waste media constitutes a biological waste that must be disinfected prior to disposal.
The Centers for Disease Control & Prevention (CDC) and National Institutes of Health (NIH) recommend that the vacuum systems used to aspirate these wastes are protected with High Efficiency Particulate Air (HEPA) filters and liquid disinfectant traps to prevent aerosolized microorganisms from being emitted into the laboratory or the system exhaust. The HEPA filter, installed inline, will isolate and confine infectious materials and prevent aerosol contamination of the vacuum pumps (see Figure). At least one collection flask is required, but two are recommended.
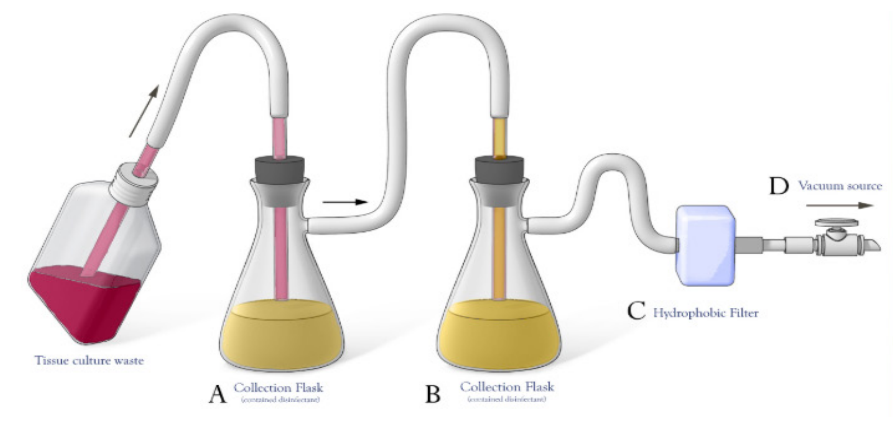
Set-up
- Aspirator is connected to collection flast and overflow collection flask (A & B)
- Flasks should contain appropriate disinfectant
- In-line filter (C) is connected to protect the vacuum line (D)
ALL vacuum lines must be protected using in-line vacuum filters (hydrophobic, PTFE, with < 0.45µm particle retention) and vacuum traps. These disposable filters are available from many scientific vendors.
|
Name and Product Number |
|
|
Vacushield Vent Device, Pall Life Sciences: Pall 4402 |
|
|
Whatman VACU-GUARD inline filter: WHAT67225000 |
|
|
MilliporeSigma Millex Filters: SLFG05010 |
|
Set up the trap(s) and HEPA filter as shown in the Figure above. The working end of this tubing is connected to a disposable plastic Pasteur pipette (e.g. Cole-Parmer # UX-34567-92), which is then used to aspirate media or other biohazardous liquid waste. A micropipette tip (e.g., P1000 tip) may be used as an alternative.
Place the vacuum flask in secondary containment (e.g., bin or tray) if outside a biosafety cabinet to hold the liquid if it is spilled or released.
Label trap flasks with a Biohazardous Waste Label and indicate the disinfectant used and date added.
NOTE: Pre-assembled systems are available from scientific suppliers, e.g. Foxx Life Sciences Vactrap #302-2101-FLS

The vacuum trap flask must contain an appropriate disinfectant, such as bleach (50mL per 500mL flask) or wescodyne (2.5mL per 500mLflask). The waste must be removed whenever the volume of the flask is ¾ full. Add additional volume of disinfectant if needed to achieve proper concentration before disposal. Mix waste and disinfectant and let sit for a minimum of 30min prior to drain disposal.
NOTE:
- If using bleach, the waste may be disposed down the sink with running water. The trap must be emptied at least every two days due to the instability of the bleach.
- If using wescodyne, then waste disposal every 3 months is acceptable if the trap not actively used. However, unless approved for sink disposal by EHS, the waste must be managed as hazardous waste through the University Lab Management System. If there are any chemical constituents, other than the bleach, or radiological constituents that are not acceptable for drain disposal, the tissue culture waste must be managed as hazardous waste.
When the vacuum suction is applied, the media flows directly into the top of the flask while the suction from the vacuum line pulls air from the side of the flask through the HEPA filter, which protects the vacuum line and the user from contamination.
In labs where the vacuum lines are used routinely, filters should be changed every six months. Otherwise, an annual change is sufficient. However, if the filter should become clogged, change the filter immediately before further use.
Adapted from Dartmouth University and University of Pennsylvania Fact Sheets